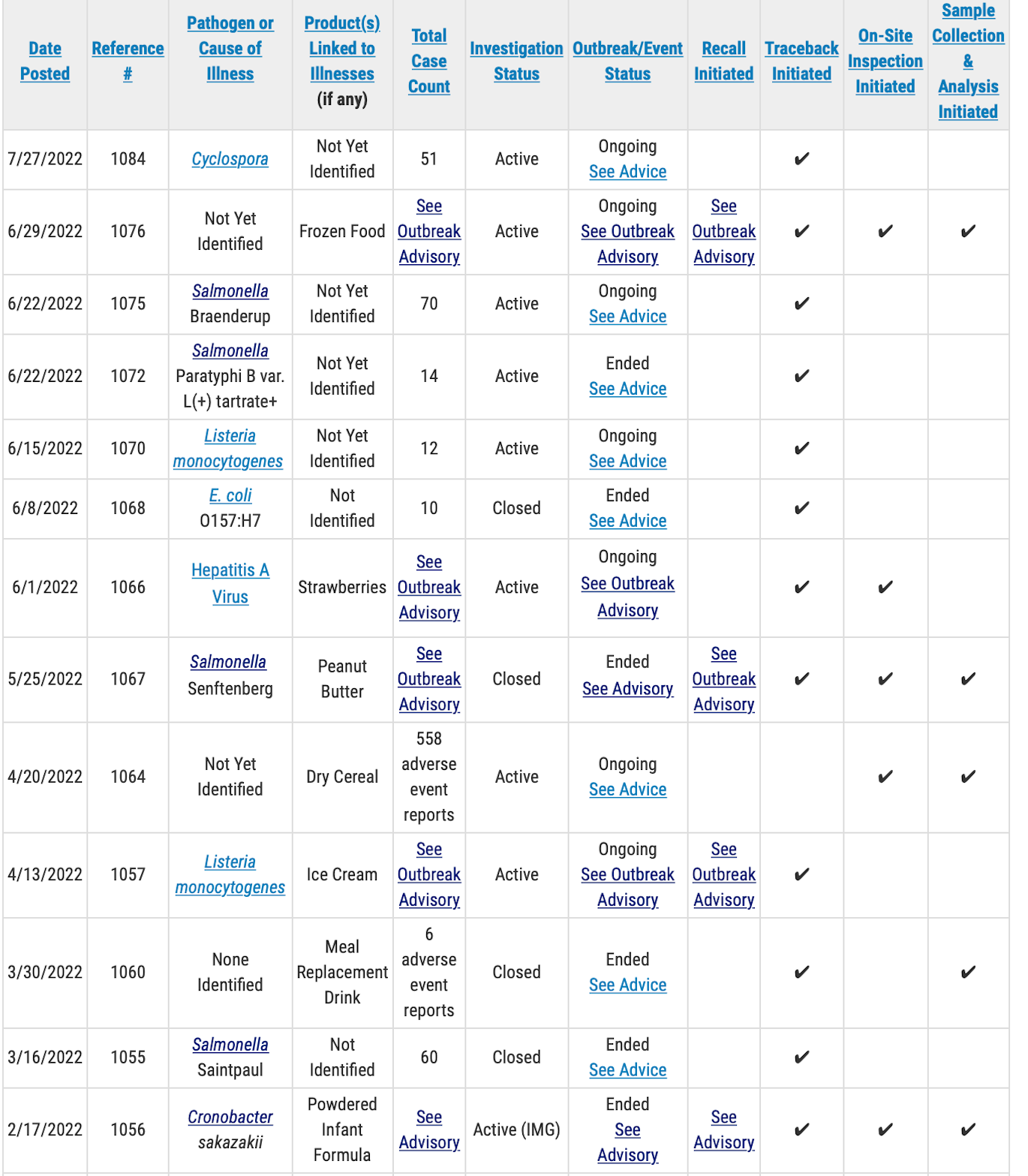
The FDA is investigating a new outbreak of dozens of infections caused by the Cyclospora parasite.
Little information has been released, but the Food and Drug Administration is reporting that 51 people have been confirmed infected. The agency has not released any specific information about the patients such as their ages or where they live.
The FDA’s outbreak information indicated that a traceback investigation has begun, but it has not reported what food or foods are being traced. The agency has not initiated any on-site inspections or testing.
In another outbreak for which the cause remains undetermined, the FDA reports that the Salmonella Paratyphi B var. L(+) tartrate+ source has not been found. The outbreak status is listed as ended with 14 people having been confirmed infected. The FDA’s investigation in ongoing and traceback efforts are under way for unspecified food or foods.
There are seven other FDA outbreak investigations underway as of July 27. They include:
- An investigation related to adverse effects associated with Daily Harvest brand frozen Leeks & Lentils Crumbles. The company has received more than 470 complaints of illnesses and as of July 14 the FDA had received 277 complaints. Some of the patients have gone into liver failure and at least 25 have had to have their gallbladders removed. The FDA is working on traceback efforts, has begun on-site inspection and product testing.
- An outbreak of Salmonella Braenderup infections in 70 confirmed patients. The cause is unknown but the FDA has begun traceback efforts. The agency has not revealed what food or foods are being traced.
- An outbreak of Listeria monocytogenes infections that has sickened 12 people. The FDA has initiated traceback efforts but has not yet reported what food or foods are being traced.
- An outbreak of hepatitis A infections traced to fresh strawberries that has sickened at least 19 people with 13 having been hospitalized. These potentially contaminated strawberries were imported from Baja California, a state in northern Mexico, and branded as FreshKampo and HEB and distributed nationwide. The FDA and the Centers for Disease Control and Prevention continue to investigate the outbreak. There have also been reports of sick people in Canada.
- An outbreak of “adverse events” involving 558 patients who ate Lucky Charms cereal. The investigation is ongoing and the FDA is conducting on-site inspections and testing.
- An outbreak of infections from Listeria monocytogenes traced to Big Olaf ice cream produced in Florida. A total of 23 confirmed patients have been reported with one death and one fetal loss. The patients are spread across 10 states and many of the sick people reported travel to Florida before becoming ill. Testing has shown Listeria in the manufacturing plant and in 16 of 17 flavors of Big Olaf ice cream. The company has been closed down by the state until further notice.
- An outbreak of infections from Cronobacter in four infants, one of whom died. The outbreak has been determined to be over by the CDC, but is it still under investigation. The babies consumed infant formula made by Abbott Nutrition’s plant in Sturgis, MI.
Additional outbreak information
The table below shows information about outbreak investigations being managed by FDA’s CORE Response Teams. The investigations are in a variety of stages. Some outbreaks have limited information with active investigations ongoing, others may be near completion. The table below has been abbreviated to show only active investigations.
A public health advisory will be issued for investigations that have resulted in specific, actionable steps for consumers to take to protect themselves, according to the FDA. Please direct your attention to those pages for the most up-to-date information on the investigation and for consumer protection information.
Outbreak and adverse event investigations that do not result in specific, actionable steps for consumers may or may not conclusively identify a source or reveal any contributing factors. Adverse event investigations rely on self-reported data. Although these reports may name a particular product, FDA will only indicate a product category in the table and will not publicly name a specific product until there is sufficient evidence to implicate that product as a cause of illnesses or adverse events. If a cause and/or contributing factors are identified that could inform future prevention, FDA commits to providing a summary of those findings.
Click here to go to the page with links to specific outbreak information.
(To sign up for a free subscription to Food Safety News,click here)









