Laboratory evidence now shows that an ongoing outbreak of infant botulism infections began in December 2023.
The Centers for Disease Control and Prevention reports that there are now 51 patients in the outbreak, all of whom consumed ByHeart infant formula. Ten of those patients became ill from December 2023 through July 2025. It was previously thought that the outbreak began in August this year.
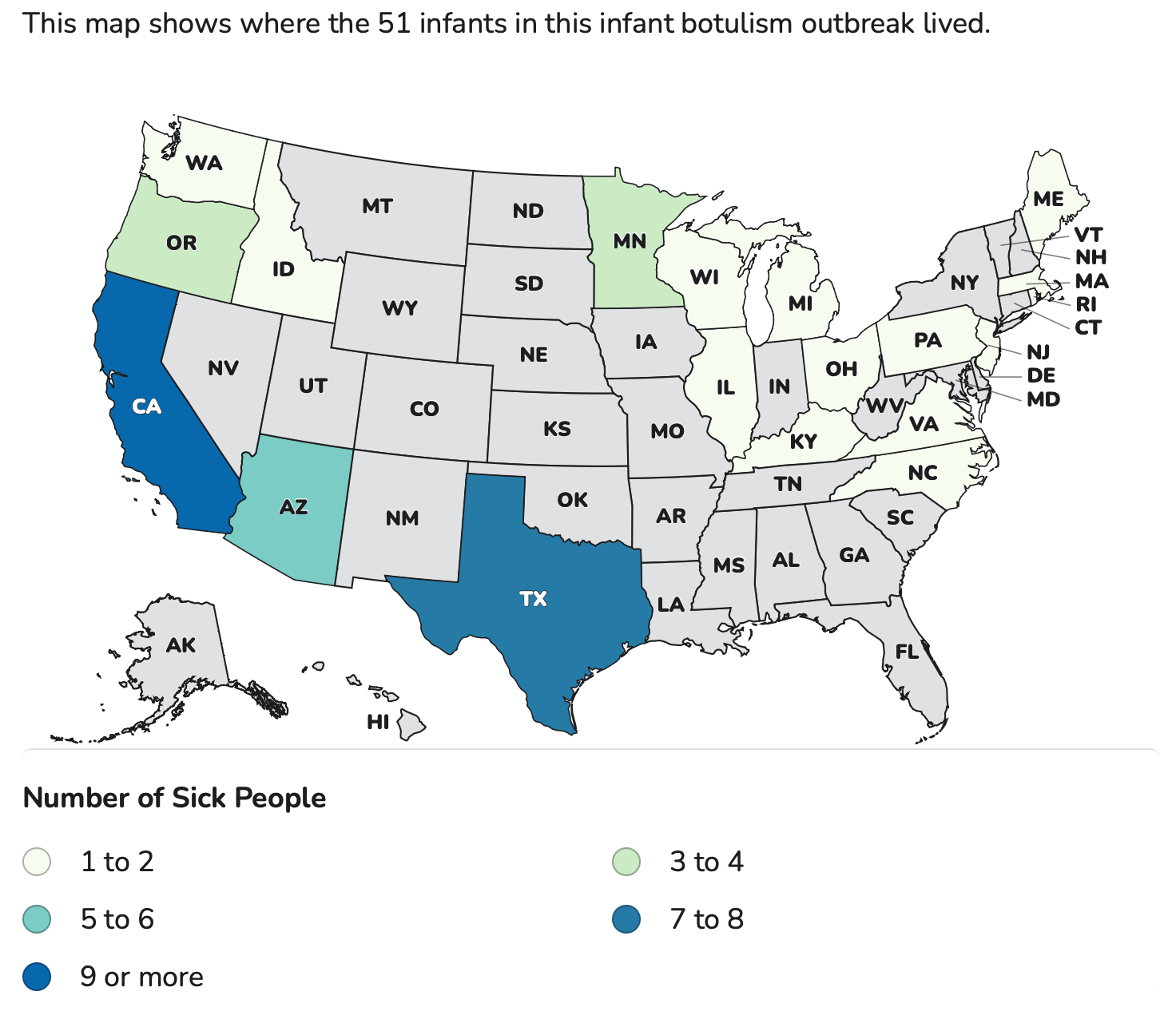
The outbreak is now spread across 19 states from coast to coast. All of the sick babies have been admitted to hospitals, but none have died. The most recent patient developed symptoms on Dec. 1. The infants range in age from 16 to 264 days old.
ByHeart first began producing powdered infant formula in 2022. It has recalled all of its formula because of the ongoing outbreak. However, the Food and Drug Administration has received reports through Nov. 26 that the formula remained for sale at retailers including Walmart and Kroger stores.
“ByHeart's and FDA's investigations into the root cause of the outbreak are ongoing, and at this time, FDA cannot rule out the possibility that contamination might have affected all ByHeart formula products,” according to the CDC’s update.
“In response, CDC broadened the case definition for the outbreak investigation to include any infant with botulism who was exposed to ByHeart formula at any time since the product's release in March 2022.”
Tests by the FDA and ByHeart have found the company’s powdered infant formula to be contaminated with Clostridium botulinum spores. The spores cause botulism poisoning.
Parents and caregivers are urged to stop using ByHeart infant formula immediately and seek medical help if their babies develop signs of infant botulism.
Symptoms include poor feeding, loss of head control, and difficulty swallowing. Infant botulism poisoning can lead to respiratory failure. If untreated, infants with infant botulism experience a progressive, flaccid paralysis that can lead to breathing difficulties and require weeks of hospitalization. Treatment with BabyBIG® is recommended for all suspected cases of infant botulism.
If you suspect your infant patient has botulism, immediately call 510-231-7600 for case consultation. Consultation is available 24 hours a day, 7 days a week.






