Years in the making, the publication of the final rule of the FDA’s Food Traceability Proposed Rule is imminent. Parts of the rule will be voluntary for industry.
The rule is among the congressional mandates in the Food Safety Modernization Act (FSMA), which was signed into law on Jan. 4, 2011.
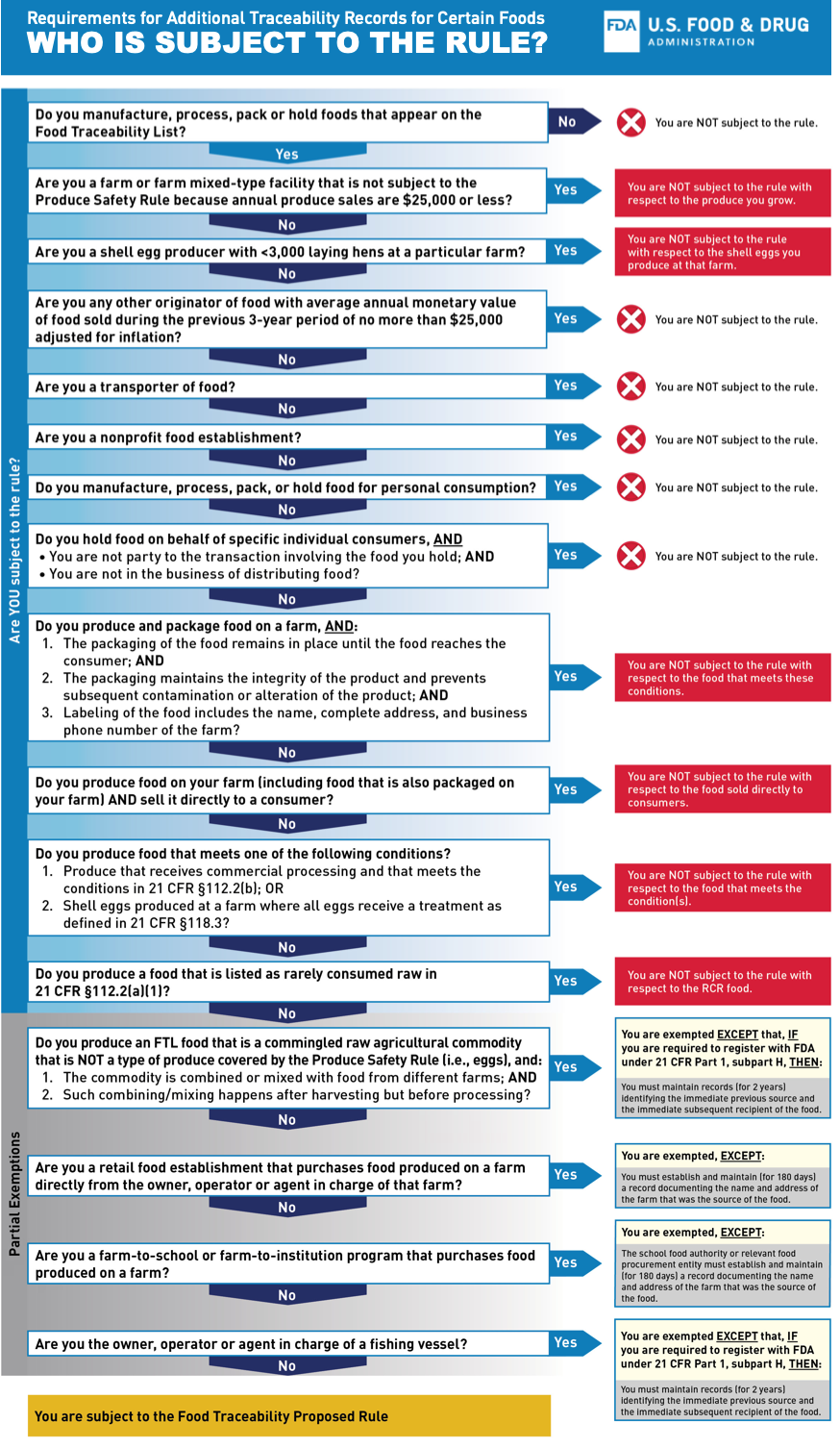
Formally named the “Requirements for Additional Traceability Records for Certain Foods,” the rule, if enacted would apply to people who “manufacture, process, pack, or hold foods the (FDA) has designated for inclusion on the Food Traceability List.”
In its notice about the proposed rule, the Food and Drug Administration said “the proposed requirements would help the FDA rapidly and effectively identify recipients of those foods to prevent or mitigate foodborne illness outbreaks and address credible threats of serious adverse health consequences or death.”
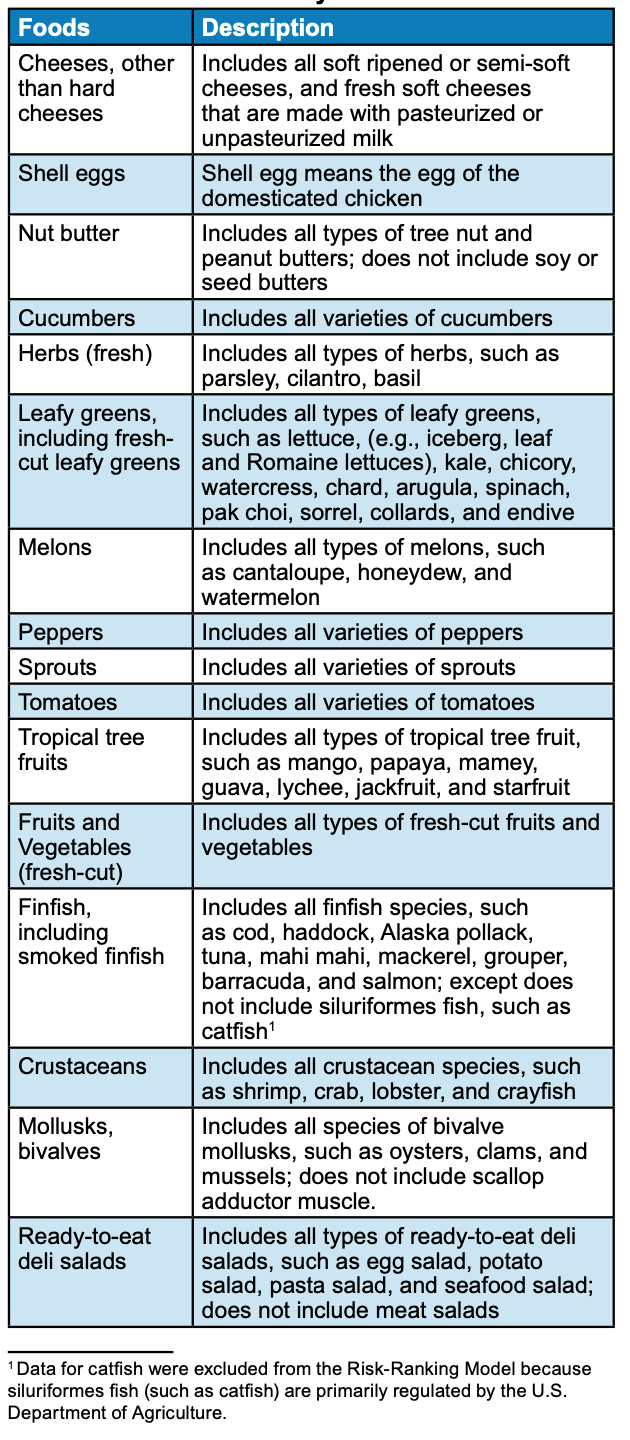
A key component of the proposed traceability rule is the “Food Traceability List” (FTL), which will specify what foods will be covered by the prosed rule. Only certain foods would be bound by the proposed rule.
“While the proposed requirements would only apply to those foods on the FTL, they were designed to be suitable for all FDA-regulated food products. FDA would encourage the voluntary adoption of these practices industry-wide,” according to the FDA’s announcement on the proposed rule.
“At the core of this proposal is a requirement for those who manufacture, process, pack, or hold foods on the Food Traceability List (FTL)to establish and maintain records containing Key Data Elements (KDEs) associated with different Critical Tracking Events (CTEs).”
Key features of the traceability rule
1. Critical tracking events
The proposed rule identifies growing, receiving, transforming, creating, and shipping as the CTEs for which records containing KDEs would be required. The KDEs required would vary depending on the CTE that is being performed. The records required at each CTE would need to contain and link the traceability lot code of the food to the relevant KDEs.
The main areas for critical tracking events are:
- Growing — For products such as fruits and vegetables, growing is generally the first step in the supply chain. In addition to the general key data elements for growing, sprout growers would be required to establish and maintain additional growing key data elements that are specific to sprouts. The FSMA gives special treatment to sprouts because of the higher danger they pose.
- Receiving — is an event in a food supply chain in which food is received by a customer, other than a consumer, at a defined location after being transported by truck or ship, or other means from another defined location. In addition to the general key data elements for receiving, “first receivers” would need to establish and maintain additional key data elements.
- Creating — is the making or producing of food on the Food Traceability List, such as through manufacturing or processing, using only ingredient(s) that are not on the Food Traceability List. Creating does not include originating or transforming food.
- Transformation — is an event in a food’s supply chain that involves changing food on the Food Traceability List, its package, and/or its label — regarding the traceability lot code or traceability product identifier — such as by combining ingredients or processing a food for example by cutting, cooking, commingling, repacking, or repackaging. Transformation does not include the initial packing of a single-ingredient food or creating a food.
- Shipping is an event in a food supply chain in which a portion of food is arranged for transport by truck or ship or other means from a defined location to another defined location at a different farm, a first receiver, or a subsequent receiver.
2. Traceability program records
In addition to requiring records of key data elements, as discussed above, the proposed rule would require anyone subject to the rule to establish and maintain traceability program records. These records are intended to help regulators understand an entity’s traceability program, and include:
- A description of relevant reference records: A firm’s key data elements might be kept on various types of reference records, such as bills of lading, purchase orders, or production logs. A firm’s traceability program records would need to include a description of the reference records on which the firm maintains the required key data elements. This description would explain where on the reference record the traceability information appears, and if applicable, a description of how reference records for different tracing events for food are linked.
- Foods on the food traceability list that are shipped: The proposed rule would require anyone who ships food on the food traceability list to keep a list of which listed foods they ship, including the traceability product identifier and traceability product description for each food. This list would be part of a firm’s traceability program records.
- How traceability lot codes are assigned The proposed rule would require traceability lot codes to be established when food on the food traceability list is originated, transformed, or created. The traceability lot code allows food to be uniquely identified throughout the supply chain. As part of a firm’s traceability program records, firms would be required to describe how they establish and assign traceability lot codes. Because of the crucial role that traceability lot codes play in the proposed rule, it is important that regulators know how a firm created and assigned these codes so that they can better understand the scope of the records they are reviewing.
- Understanding data in required records The proposed rule would require a firm’s traceability program records to include any other information needed to understand the data within their traceability records, such as internal or external coding systems or classification schemes, glossaries, and abbreviations. This will help regulators understand the terminology, methods, and systems a firm uses in its traceability operations.
3. Additional Requirements
The proposed rule would also require that:
- Records are maintained as either original paper records, electronic records, or true copies; they all must be legible and stored to prevent deterioration or loss.
- Traceability records are provided to FDA as soon as possible but no later than 24 hours after a request is made.
- An electronic sortable spreadsheet containing relevant traceability information be provided to the FDA within 24 hours of a request when necessary to assist the FDA during an outbreak, recall, or other threat to public health.
To view frequently asked questions about the proposed food traceability rule, click here.
For a discussion of exemptions to the rule, click here.









